Welcome to our Regulatory News Portal
Global Chemical Regulatory Insight
The Latest News
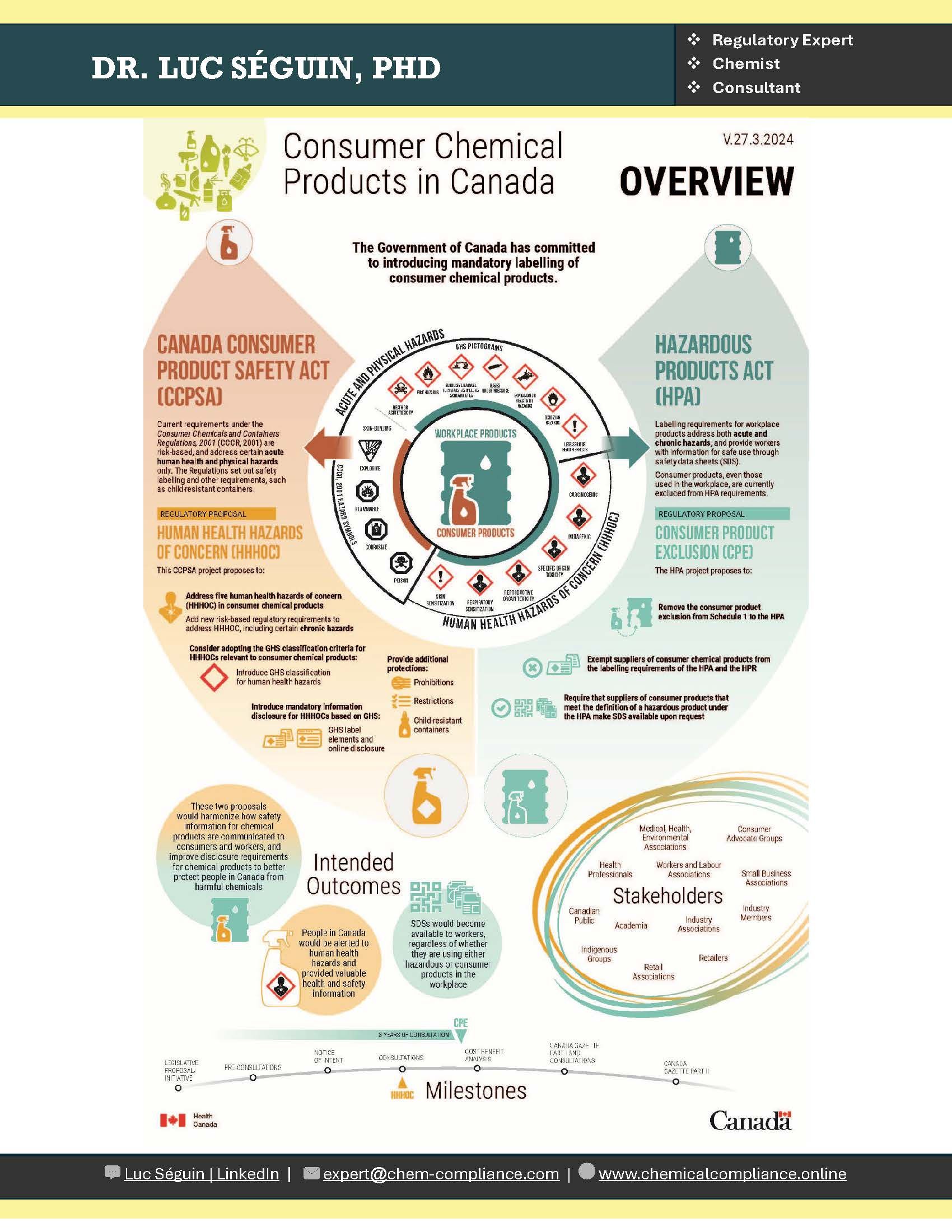
End of transition period for the amendments to the Hazardous Products Regulations
The 3-year transition period ends on December 14, 2025, for updating product classifications, Safety Data Sheets (SDSs), and labels to meet the amended Hazardous Products Regulations (HPR).
Starting December 15, 2025, all hazardous products must have hazard classifications, SDSs, and labels (if applicable) that fully comply with the amended HPR. In addition, any SDSs or labels submitted with claims for exemption under the Hazardous Materials Information Review Act on or after December 15, 2025, must comply with the amended HPR. If these documents are prioritized for in-depth compliance review, they will be assessed according to the amended HPR. Claimants who submitted SDSs and labels based on the former HPR prior to the end of the transition period may be required, at Health Canada's request, to provide a revised SDS and/or label based on the amended HPR following the end of the transition period.
Health Canada acknowledges stakeholder concerns regarding the alignment of implementation timelines with the U.S. Occupational Safety and Health Administration (OSHA), particularly with respect to SDSs and labels for mixtures. Health Canada understands the importance of regulatory consistency for Canadian companies and recognizes the efficiencies of harmonized requirements and timing.
Health Canada remains committed to the objectives of the Canada-U.S. Regulatory Cooperation Council, including mutual alignment of GHS implementation in both countries. Health Canada therefore plans to take the following approach to compliance with, and enforcement of, the amended HPR requirements:
- Until July 19, 2027 (the U.S. implementation deadline for mixtures), Health Canada will focus on compliance promotion with regulated parties; and,
- From July 19, 2027, onwards, compliance and enforcement will follow a risk-based approach as per standard departmental and program guidance.
Throughout this period, Health Canada may take measures to induce or compel compliance with the HPR, if warranted.
You can read more about this and other efforts that Health Canada is taking to reduce regulatory red tape and support robust economic growth in Canada in the Report on Red Tape Reduction published on September 8, 2025.
GHS Revision 11
The United Nations has just released Revision 11 of the GHS (September 12, 2025). You can access the PDF versions in French, English, and Spanish on the website link below.
Introducing our publication "The Regulatory Secrets"
I'm pleased to launch this dedicated space for sharing critical regulatory information with my clients and industry colleagues.
After years of helping companies navigate the complexities of chemical compliance, particularly within Canadian frameworks, I recognized the need for accessible, timely insights on regulatory developments.
This publication will deliver expert analysis on emerging regulations, compliance strategies, and industry best practices—information you can trust from a Canadian consultant with extensive experience in both domestic and international chemical regulatory landscapes.
I invite you to frequent this page for regular updates designed to help you stay compliant while turning regulatory knowledge into a competitive advantage.